

Of course, Keynote-799 is an uncontrolled study, and as with the Cityscape and Checkmate-9LA analyses the comparisons are across different studies that possibly recruited differing patient populations. Keytruda already has a first-line stage III NSCLC label, courtesy of the Keynote-042 trial, but only in PD-L1 ≥1% patients who are not candidates for chemoradiation. But the important point is that in Keynote-799 Keytruda was given with chemoradiation, whereas Pacific enrolled subjects who had already been given chemoradiation and had not progressed thus, the potential is for Keytruda to establish a niche in a setting before that in which Imfinzi is approved. In Pacific Imfinzi showed a six-month OS of about 92% and ORR of 28.4%. The threat of other checkpoint blockers here has been looming, and Asco reveals the first data from Merck’s Keynote-799 trial, in which a Keytruda regimen yielded a 56.6% remission rate, and six-month OS of 94.8%. And the toxicity burden is considerable: in Checkmate-9LA 47% of patients on the combo experienced grade 3 and 4 toxicities, versus 38% for control.Ī separate NSCLC setting is pre-metastatic, stage III disease, where Astra’s Imfinzi is carving out a niche thanks to approval based on its Pacific trial, notwithstanding doubts about its benefit in PD-L1 non-expressers. This looks promising for approval, but like Roche’s Tigit it hardly challenges Keytruda, whose chemo combo study Keynote-189 yielded a 51% reduction in risk of death versus chemo alone. Indeed, the triple combo reduced risk of death by 31% versus chemo alone (p=0.0006), an interim analysis reveals longer follow-up shows an improving picture, with median OS of 15.6 versus 10.9 months, and a 0.66 hazard ratio.
CHECKMATE 9LA STUDY DESIGN TRIAL
The Asco abstract reveals the first efficacy data from this second trial since it was said to have succeeded last October in improving overall survival. In Bristol's case, Opdivo is due a double US FDA verdict on use in 1st-line NSCLC, first, by tomorrow, as a Yervoy combo based on the controversially overhauled Checkmate-227 trial, and then by August 6 on the basis of Checkmate-9LA, a study combining Opdivo with Yervoy and chemotherapy. Source: Asco, company releases & product labels. Cross-trial comparisons: Roche's Tigit data in context
CHECKMATE 9LA STUDY DESIGN PLUS
But in and of itself a Tecentriq plus tiragolumab combo looks no better than Keytruda in its already approved first-line NSCLC use Arcus, a biotech Tigit player that has traded up strongly in tandem with Roche’s Tigit plans, opened off 11% this morning. On the plus side Tigit blockade appears to add no toxicity. Yet Tigit blockade appears to add nothing, providing a clue to why Cityscape enrolled ≥1% PD-L1 expressers, and why Roche’s phase III Tigit trial, Skyscraper-01, enrols ≥50% subjects. The first point is particularly relevant, as it is PD-L1-low subjects where the efficacy of PD-(L)1 blockade is in need of boosting. The bad is that this is strongly driven by the highest (≥50%) PD-L1 expressers, and the combo numbers only look good because Tecentriq alone performs particularly badly. The good news for Roche is that addition of Tigit blockade to PD-L1 shows an additive effect in terms of response rates and median progression-free survival. Cityscape is only a phase II trial, but it has a robust design: tiragolumab plus Tecentriq versus Tecentriq alone. Tigit became a closely watched immuno-oncology target after Roche began a wide-ranging clinical programme with tiragolumab this year, but until Asco data supporting it had been scant. And Merck could do some catching up of its own: a study in stage III NSCLC shows that Keytruda could have Astrazeneca’s Imfinzi in its sights. The keenly awaited Cityscape trial of the anti-Tigit MAb tiragolumab, for instance, shows why Roche is investing heavily in the project, though its real-world relevance seems marginal.įor Roche the most important thing is to find a way of catching up with Merck & Co’s Keytruda – a similar consideration to Bristol-Myers Squibb, which has unveiled its Checkmate-9LA study, meant to support approval of Opdivo plus Yervoy and chemo.
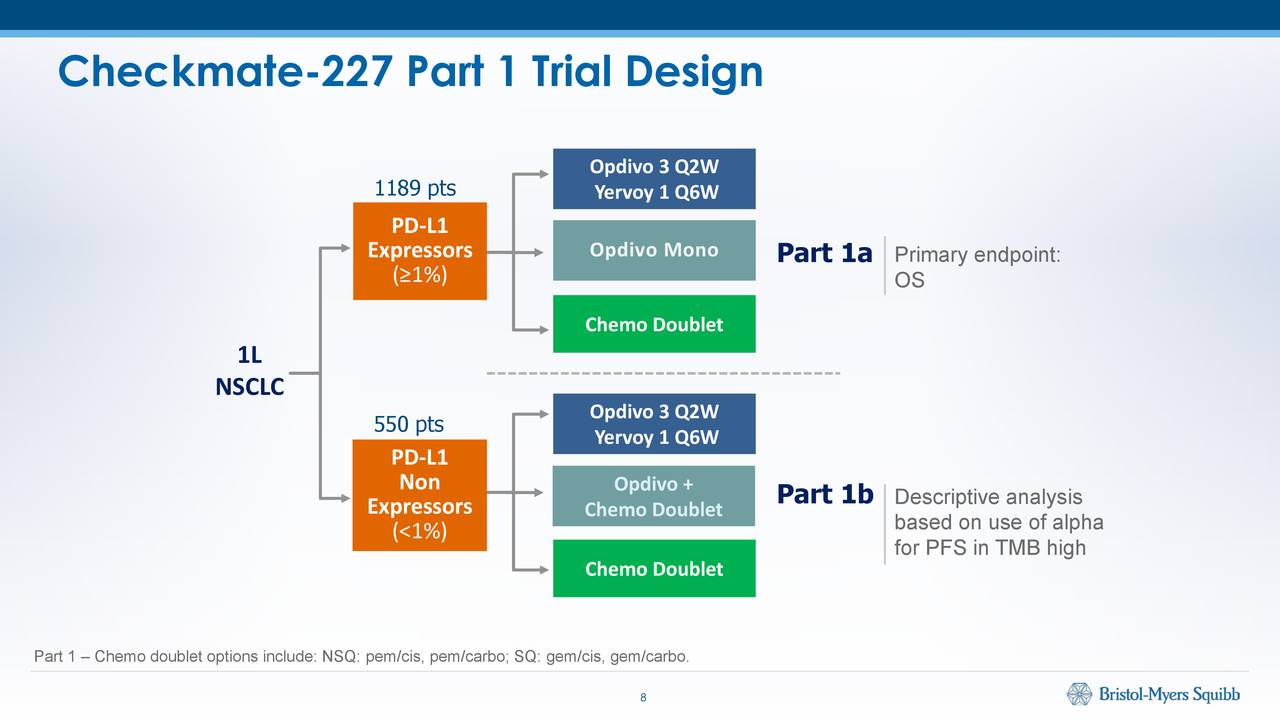

Last night’s Asco abstract dump has revealed several results of interest to companies pursuing first-line non-small cell lung cancer.


 0 kommentar(er)
0 kommentar(er)
